The views expressed here are solely those of the author and do not necessarily represent the views of FreightWaves or its affiliates.
The logistics of delivering a COVID-19 vaccine to all Americans will be historic. Major Gen. Christopher J. Sharpsten, the deputy director for supply, production and distribution for Operation Warp Speed (OWS), provided a detailed breakdown of this logistics mission. The Q&A below explains the broader strategy of OWS to accelerate the development, manufacturing and distribution of COVID-19 vaccines and therapeutics.
LL: Thank you for taking the time to break out this mission. In a recent Chamber of Commerce webinar, Tanya Alcorn, Pfizer’s vice president for biopharma global supply chain, explained they are using their own distribution plan. Can you explain how this independent plan will be folded into OWS?
CS: The Operation Warp Speed distribution strategy uses a centralized distributor, McKesson, for all vaccine candidates except Pfizer. This is intentionally done due to the company’s internal capability for nationwide distribution and requirements for ultra-cold transport and storage.
Distribution for Pfizer’s vaccine will be closely coordinated and managed by Operation Warp Speed for the doses purchased by the U.S. government. It will include distribution from the manufacturing site direct to administration sites to minimize time in transit.
Pfizer has embedded a logistics liaison within the Operation Warp Speed operations center and is working hand-in-hand to plan distribution for their vaccine.
LL: We have heard President Trump, in his rallies and in the first presidential 2020 debate, that the vaccine deployment is “logistically all set up” using soldiers to deliver “200,000 a day.” But we know it is not really that simple. What kind of planning and preparation has gone into this?
CS: We really view this as a “whole-of-America” approach. We know we would not be able to accomplish this mission without the unparalleled expertise of HHS scientists and CDC groundwork, as well as what we consider the DOD’s expertise in planning and logistics. So that, combined with the ingenuity, the understanding, the innovation of American industry as well as academia, we think this is a magical set bringing all this together, allowing us to achieve this mission in a really unprecedented time.
LL: Moncef Slaoui, scientific head of Operation Warp Speed, recently said most Americans may have access to a COVID-19 vaccine by early this spring, one which could potentially immunize them by June. What are you seeing from a logistics perspective?
CS: This is a function of when the vaccine will be approved and how much production has taken place. Until that happens, based on the estimates we have, I would not disagree with what Dr. Slaoui has stated publicly that all Americans could be immunized by June.
LL: Is there an approximate number on the amount of public and private companies working in this logistical supply chain?
CS: In addition to McKesson, UPS Healthcare and FedEx are the major subcontractors of this mission. UPS Healthcare and FedEx can also sub out to other contractors.
You have seven manufacturers. Six have advance orders in production and have 22 manufacturing plants in 13 states and three countries — the U.S., Canada and the Netherlands. You also have subcontractors providing logistics support, helping secure both the upstream supply chain raw materials as well as the making of the vaccine supporting equipment.
LL: The “cold/cool chain” is an extreme delicate balancing act because it requires precise control of the vaccine’s temperature. What critical pieces are supporting this chain to ensure the packages and containers will hold them at their critical temperatures?
CS: The safety and the efficacy of a vaccine will be determined through science and data. This not only includes the clinical trials but also the stability studies being run by the manufacturers. Both will be a part of the FDA review process.
Once we have clear guidelines from the FDA, they will be published under the Emergency Use Authorization. Once we move into the distribution and administration phase in the delivery of the vaccine, we intend to apply that same discipline with science and data to the distribution efforts to each company being used. We will use the existing pharmaceutical companies that routinely deliver biologic and drug products and leverage their expertise in maintaining proper handling and cold chain management.
The efficacy assurance requirement of the vaccine will be broken down into two different perspectives. The first one is from the manufacturing and shipping point of view.
The pharmaceutical industry routinely deals with a multitude of products, cold chain products. We are working in conjunction with those companies to understand the current technology, the current techniques and the procedures. In all cases we will be using passive monitoring devices on each vaccine shipment. There will be monitoring of temperature of all vaccine products throughout the distribution system.
LL: What kind of temperature monitoring?
CS: OWS will monitor the Pfizer vaccine with a passive technology device created by Pfizer. The other products, if approved by the FDA, will use a different passive monitoring device by OWS.
If the product falls out of tolerance while it is in the distribution channel, that container will essentially be quarantined. We’ll monitor the inventory of vaccine product from the manufacturer to the administration site through our database system. If a shipment potentially goes bad, we’ll have a contingency plan in place to make up for that loss.
These devices monitor the temperature over time and when it gets to a point where the package is out of tolerance, a red arrow will start flashing.
LL: The vaccines in development vary in temperature moderation. There have been concerns about the supply of dry ice. Is there reason for concern?
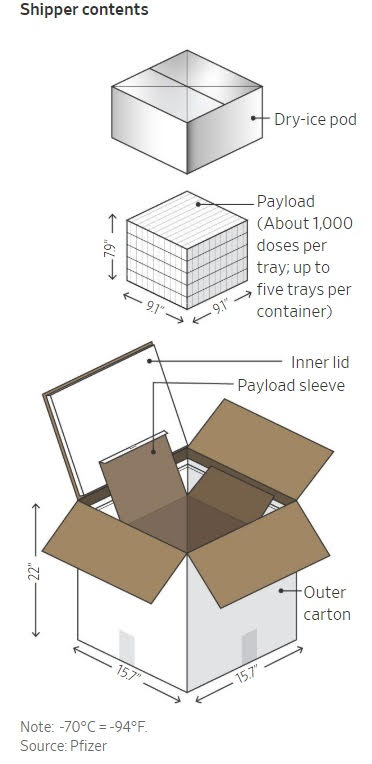
CS: The Pfizer vaccine candidate is the only Operation Warp Speed contracted product that would require ultra-low-temperature handling. Pfizer is also making its own dry ice.
Pfizer developed a special shipping box that uses dry ice to hold between 975 doses and 4,875 doses of vaccine. This shipping container is essentially 20 inches by 20 inches by 24-inch box with different thermal layers in it, and these shippers have what’s called a passive time-temperature indicator to maintain cold-chain quality control.
According to tests by Pfizer, unopened, these Pfizer shipper boxes maintain minus-80-degree Celsius for up to 10 days. Operation Warp Speed received a sample of the shipper at headquarters and has been going over the product daily. We are picking it apart and putting it back together to make sure we understand it.
LL: There are FAA rules on how much dry ice can be on a plane. What kind of planning is going on associated with these rules?
CS: For dry ice, we are really only talking about the Pfizer product so it is only one of six vaccine potential candidates that could come out.
During an OWS weekly distribution working group call with McKesson, UPS and FedEx, we learned the FAA has established a dry ice shipping working network. FedEx is a part of that working network.
We rely on UPS and FedEx expertise to get this done. If they subcontract out and decide to use crews from United, Delta, etc., it would be incumbent by them to ensure that they adhere to those FAA regulations. The U.S. government has not stipulated how that should go.
LL: What is the second problem set OWS is focused on?
CS: The second problem set Operation Warp Speed is focused on is vaccine administration. OWS is concurrently planning with the states and territories and major metropolitan areas. There is a total of 64 jurisdictions, each with hundreds of administrative sites, across the United States.
Some states are moving at an exceptional pace and are doing great things. With other states, where they may lack necessary expertise on a pandemic response, we are providing additional assistance to them. We are required. So we really do view this as a whole-of-America approach. We need to make sure they have sufficient cold-chain capacity at those sites to receive, store and administer the vaccines. The needs of these sites fall under two certain criteria: how much product OWS needs to send to them and how fast can they can administer those vaccines to patients.
It is not necessarily that the federal government is telling a state or a major city how to do something. It’s about understanding their process so that we can ensure we deliver to them in the appropriate manner with the appropriate product. It’s really a hand-in-hand development of their plans so we can optimize their delivery and really increase uptake of shots to the American people.
LL: Will there be enough ultra-cold freezers to house the Pfizer vaccine at these administrative sites if that is the vaccine that is submitted and approved?
CS: We are reviewing the state response plans and are taking down their asks so we can assist them in determining the best locations for vaccination administration sites. Freezer capacity at minus 20 C and minus 80 C is a priority for these sites. So we are working with jurisdictions on how to best implement that Pfizer vaccine.
Because of the Pfizer product, the way it is shipped and the way it must be stored, we’re really focusing on this Pfizer product as a product where we would essentially bring the people to the vaccine as opposed to bringing the vaccine to the people.
LL: Can you give a possible scenario on the methodology being used to determine if an administrative site needs a deep freezer or not?
CS: The minimum shipment of a Pfizer shipment is 975 doses. So once the vaccines thaw, they can essentially be kept at 2 C to minus 8 C for five days. Most administration sites can do that. So if they can administer 975 doses within five days, ultra-low-temperature cold storage is not really a problem or requirement because they will go through the doses before the shipment expires.
LL: What about the logistics of the other vaccines?
CS: If a more stable vaccine, one that could be stored at 2 C to minus 8 C with stability of up to three months, that vaccine could be easily distributed far and wide to small doctors offices, etc. J&J’s vaccine would require storage to be stable for two years at minus 20 C and for upward of three months in the 2 C to 8 C range used to store many biologics.
Moderna and AstraZeneca have stated they expect their vaccine to require storage at minus 20 C/minus 4 Fahrenheit. Most commercial freezers can reach that temperature.
LL: Another concern in the logistics chain is the fear of a lack of freezer trucks and the trickle-down impact it could have on other medicines and food supply. Do we have enough freezer trucks for this mission so both medicines and food supply are not impacted?
CS: No. Based on their routine industry calls every day, both in industry or related industries, it is hard to anticipate but we do not expect any hiccups in the movement of other drugs or food industry channels.
As far as distribution capacity, we assessed it with our industry partners, McKesson, FedEx and UPS Healthcare, and they tell us that we are in a good position. These companies can scale up or scale down capacity based on requirements and orders and they don’t foresee any problem. FedEx and UPS think there might be a crunch on routine airlift capability during the holiday season but since COVID-19 products are receiving a higher priority of service, they have guaranteed essentially white-glove service, that vaccine delivery will definitely not be impacted. Matter of fact, we are guaranteeing almost 24-hour delivery to administration sites once we hit the main hubs at Nashville and Louisville.
Our calls include Americold Logistics, which has 22% of the domestic market for the cold-chain storage for food. We have also had a cryogenics industry call discuss their ability to produce cryogenics freezers that could go down minus 160 and minus 90. Other ongoing calls we have include the Global Cold Chain Alliance.
While yes, they are more food focused, we asked them these questions because we know this part of the industry is important to us. We are doing deep market research with them. We are having calls with the leading companies and professional associations out there to better understand the market and which capabilities exist.
LL: In addition to the vaccine, we will also have the concurrent movement of the associated ancillary kits (syringes, needles and alcohol swabs). The flood of these products into the trucking system has sparked concerns of a possible trucking bottleneck. Are you concerned about any bottlenecks?
CS: We are confident in the logistics flow. In terms of any impact to the other pharmaceuticals or drug industry as well as the food industry, no, we are not anticipating any issues at this time.
LL: I don’t have to tell you about the bad guys of the world. We have read numerous stories on hacking attempts for the vaccine data itself. What kind of planning are you doing to ensure the vaccine doesn’t fall into the wrong hands?
CS: As career Army, we see bad people behind every rock. This is a high priority for us. This team is embedded from members of not only the military but also members of Health and Human Services that do this on a daily basis. I would tell you at Warp Speed, all modes of transportation are important and they will be secure through a whole-of-government, whole-of-industry approach.
Our security efforts incorporate numerous interagency partners, both local and federal. They do include the U.S. Marshals Service that is on board here with us at Operation Warp Speed for transportation security. The whole-of-government approach we are using leverages each organization’s inherent capabilities in accordance with their authorities. Our intent is to leverage all of that to ensure that we see the threats that are out there and then we mitigate those risks in advance of us actually moving product over the road movement or through the air field movement as well.
The only thing we care about is making sure every American has the opportunity for a COVID-19 vaccine. Who gets the credit for it? We are not interested in that at all. We just want to make sure it happens.











